Product Regulation
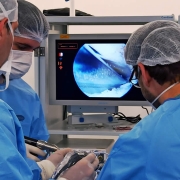
Mazzaferro Medical aims to gain FDA 510K clearance on its products for Sports Medicine (endobuttons, all suture anchors, needled round and tape sutures) on the 1st semester of 2021. With a robust regulatory system and ISO13485:2016 Quality Management, Mazzaferro Medical is a global player on the Surgical Sutures and Sports Medicine markets providing biocompatibility reports according to ISO10993. Being also certified ISO56002:2019 is very agile and consistent throughout the Innovation process.