Tag Archive for: Innovation

Coming Soon: Mazzaferro Medical FDA510K Clearance
News
Mazzaferro Medical aims to acquire FDA 510K clearance on the 3rd quarter of 2021 on its assembled products for Sports Medicine such as fixed loop endobuttons, adjustable endobuttons, all suture anchors and needled and not needled round and…

Brendan Brogan: new North America Sales Manager of Mazzaferro Medical
NewsMZF4 has just hired Mr. Brendan Brogan as the new North America Sales Manager of its Mazzaferro Medical unit.
This is another important step, after having invested more than USD 10MM in a brand new facility to produce MAZFORCE - the high…
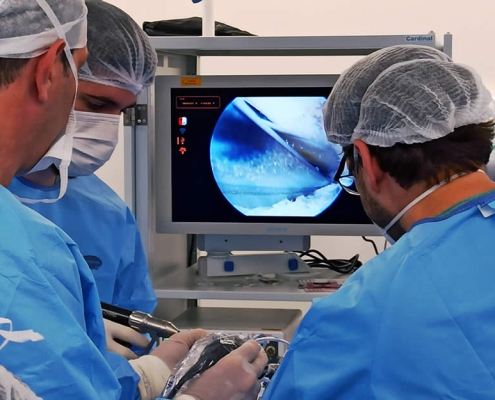
Tailor-made endobuttons
News
We envision potential disruptive innovation in Sports Medicine. Our Technical Advisory Board members, led by Dr. Castropil, on ACL surgery in the picture below looks for adjustable and fixed loop endobuttons improvements.
Mazzaferro Medical:…

Technical Advisory Board to promote innovation in Sports Medicine
News
Mazzaferro Medical, a Sports Medicine OEM - focused company, innovates creating a Technical Advisory Board (TAB) with doctors, engineers and those responsible for R&D and manufacturing so that products are designed from conception to final…

A Global Company
News
Mazzaferro Medical supplies surgical sutures and textile medical devices for Sports Medicine to customers from over 40 countries rendering a customized in company R&D, design, prototyping, and innovation service.
We are modeled to…

Our Value Proposition
News
"We are a no trade-off and passionate company. Speed, Price, Regulation, Product Design, Innovation: our mindset. The fastest prototyping process: our commitment to be your trusted OEM partner on complex textile medical devices for Sports…

Our Business Model
News
We are not just a company... we are passionate people aiming to connect with your soul. Since our beginning back in 1953 we are a B2B made to order company with strong chemical, textile and biomedical engineering background promoting highly…

Mazzaferro Medical enters US Market
News
Mazzaferro Medical has transferred its headquarter to Miami (FL). This movement is coherent with the strategy to enter the most relevant Sports Medicine Market.
The 1st step will be directed to render Commercial, R&D, Innovation and…

Product Regulation
NewsMazzaferro Medical aims to gain FDA 510K clearance on its products for Sports Medicine (endobuttons, all suture anchors, needled round and tape sutures) on the 1st semester of 2021. With a robust regulatory system and ISO13485:2016 Quality Management,…
